Vero Cell Zulassung - Eu Medicines Agency On Twitter Covid19vaccines Where Are We Now Ema Starts A New Rollingreview For Vidprevtyn Sanofi Pasteur No Marketing Authorisation Application Currently Under Review Https T Co T5aeifknsk
3 affordability and viability. Formerly called Cercopithecus aethiops this group of monkeys has been split into several different speciesThe lineage was developed on 27 March 1962 by Yasumura and Kawakita at the Chiba University in Chiba Japan.

Astrazeneca Nebenwirkungen Wirksamkeit Alle Infos Zum Corona Impfstoff Berliner Morgenpost
Kawakita at the Chiba University in Japan1.

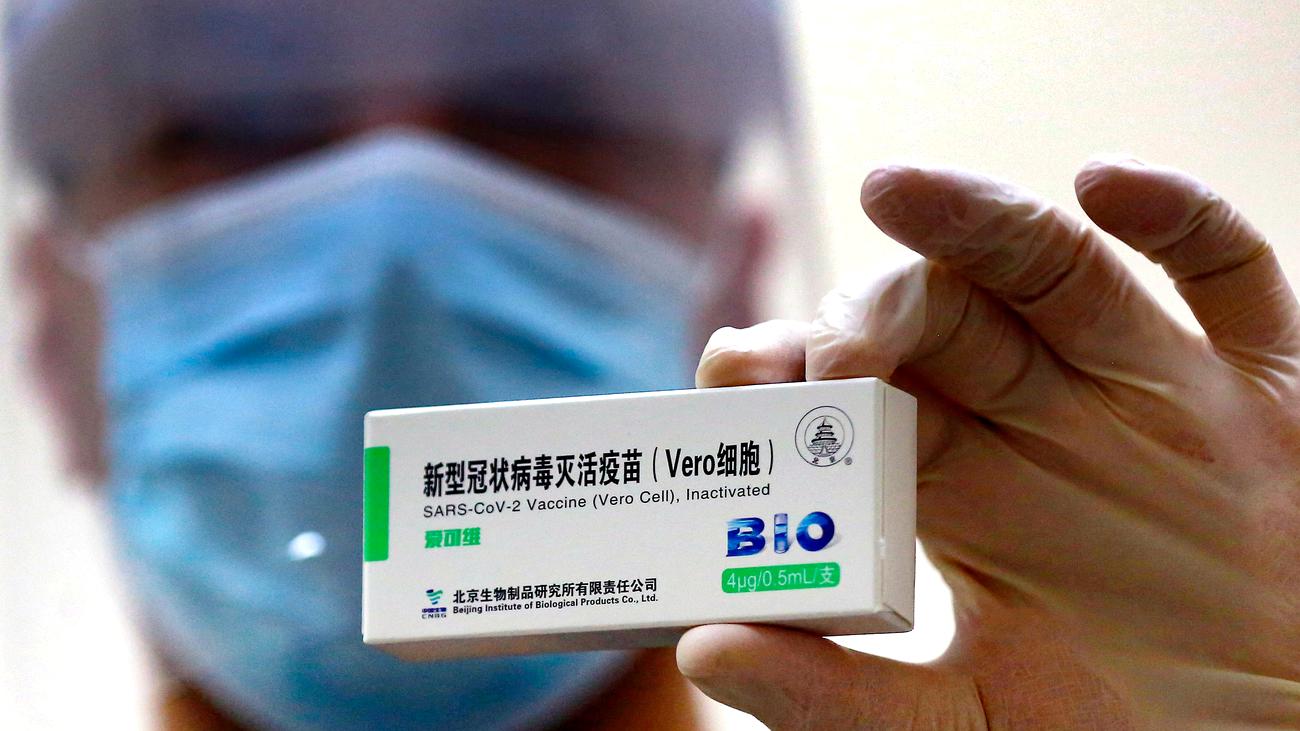
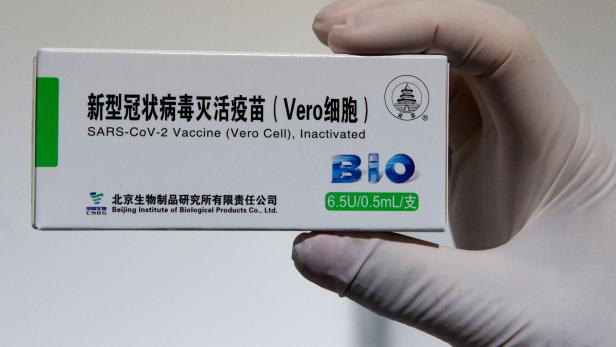
Vero cell zulassung. The vaccine called Vero is based on an inactivated. After 24 hrs of VERO cell line thaw we observed granules in the cell cytoplasm attachment VERO_24hrs. The COVID-19 Vaccine Vero Cell Inactivated CoronaVac is an inactivated vaccine against coronavirus disease 2019 COVID-19 which stimulates the bodys immune system without risk of causing a.
Sinopharmwuhan institute of biological products. Disodium hydrogen phosphate dodecahydra te sodium dihydrogen phosphate monohydrate sodium chloride DESCRIPTION CoronaVac is a milky-white suspension. Sinopharm hat eine zulassung von vero bei der.
However Vero cells are the most widely accepted among others. This review introduces briefly the concepts of advanced cell culture-derived influenza vaccine production and highlights the advantages of these vaccines in terms of efficiency speed and immunogenicity. Vero ECACC catalogue no.
The information that the vero cell sinopharm vaccine is stored in fmс 8 at 17 degrees is false. On December 29 2020 Sinopharm reported 79 efficacy in an interim evaluation. Sinovac is a Beijing-based pharmaceutical company focused on the research and development of vaccines and its Vero Cell jab gets its name.
84113001 Cell line history The original Vero cell line was established from the kidney of an African green monkey in 1962 by Y. The cells are in Eagles MEM with 10 FBS 2 mM L-Glutamine and 50 ugml Gentamycin. KAZINFORM The first batch of Sinopharms Vero Cell vaccine has.
2 trials in 1 country. Vero Cell Sinopharm DDA. Das vakzin von sinopharm mit dem namen vero.
Der Covid-19-Impfstoff Vero Cell enthält auch. Sinopharm die zulassung für seinen impfstoff mit dem. EMAs human medicines committee has started a rolling review of COVID-19 Vaccine Vero Cell Inactivated developed by Sinovac Life Sciences Co LtdThe EU applicant for this medicine is LifeOn Srl.
Vero cells are a lineage of cells used in cell cultures. Vero cell vaccine sinopharm or sinovac. The CHMPs decision to start the rolling review is based on preliminary results from laboratory studies non-clinical data and clinical studies.
Vero Cell Sinopharm Efficacy Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News. It is currently one of the most used continuous cell lines in the world and has been cited in over 10000 research publications. COVID-19 Vaccine Vero Cell Inactivated COMPOSITION Active ingredient.
This vaccine is approved. EUA of covid 19 vaccine Vero cell inactivated. Sinopharm die zulassung für seinen impfstoff mit dem.
Making vaccines using Vero cells as a matrix has improved the safety and controllability of product quality. Inactivated SARS-CoV-2 Virus CZ02 s train Adjuvant. Vero cell vaccine covid-19.
So far Vero is the only Chinese vaccine for which the manufacturer has published official data. The Vero lineage was isolated from kidney epithelial cells extracted from an African green monkey Chlorocebus sp. Vero Cell is produced by Beijing Bio-Institute of Biological Products Co Ltd a subsidiary of China National Biotec Group CNBG.
Non Replicating Viral Vector. Nepal has granted emergency approval to the chinese vero cell vaccine given the active caseload that stands at 277944 on monday while 273240 people have recovered in nepal. Der Impfstoff enthält totes SARS-CoV-2-Virus das inaktiv ist und die Krankheit nicht verursachen kann.
Vero cells are lineages of cells used in cell cultures that firstly was isolated from kidney epithelial cells extracted from an African green monkey Cercopithecus aethiops. So far Vero is the only Chinese vaccine for which the manufacturer has published. All documents required for emergency use listing EUL of Covaxin have been submitted to WHO as of July 9.
Approved in 45 countries. The lineage was. Sinopharms Vero Cell CanSino Bios Ad5-nCov CureVac AGs CVnCoV Beneficiaries of Covaxin in India have raised serious concerns about their travel plans being jeopardized if the vaccine is not approved by the WHO.
Serum Institute of IndiaCovishield OxfordAstraZeneca formulation Phase 1. EMA is not involved in advising on travel requirements in the European Union EU such as vaccination quarantine or testing for travellers. The main application area of Vero cell culture medium technology was the development and production of viral vaccines.
Decisions about which COVID-19 vaccines are included for example in the EU Digital COVID Certificate are taken by the EU Member StatesEMA is in charge of the scientific evaluation of vaccines for EU marketing authorisation. The Sinopharm COVID-19 vaccine vero cell inactivated vaccine resource includes key information on the vaccine specific requirements. In the future Vero cells may be used in the development and application of current coronavirus vaccines COVID-19.
Paul Ehrlich Institut Meldungen Start Des Rolling Review Verfahrens Fur Sinovacs Ganzvirusimpfstoff Covid 19 Vero Cell Inactivated Bei Der Europaischen Arzneimittelagentur

Astrazeneca Corona Impfstoff Nur Fur Jungere Ema Bringt Begrenzte Zulassung Ins Spiel Manager Magazin

Impfstoffe Who Erteilt Sinopharm Notfallzulassung Wiener Zeitung Online

Notfallzulassung Fur Chinesischen Corona Impfstoff Aktuell Asien Dw 07 05 2021

Sinopharm Und Co Was Konnen Die Chinesischen Impfstoffe Berliner Morgenpost
Chinas Coronavac Zwischenergebnisse Fur Kinderimpfung Mdr De
Eu Arzneimittelbehorde Startet Prufung Von Chinesischem Corona Impfstoff

Who Erteilt Sinopharm Notfallzulassung
Who Zulassung Fur Chinesischen Impfstoff Wohl Doch Registrierung In Der Gastro Kurier At

Sinovac Ema Pruft Chinesischen Covid 19 Impfstoff Pz Pharmazeutische Zeitung

Fragen Und Antworten Zur Corona Impfung In Der Eu Eu Kommission

Mogliche Zulassung Fur Die Eu Ema Startet Prufung Des Corona Impfstoffs Von Sinovac Aus China Svz De

Sinopharm Soll Zu 79 Prozent Wirksam Sein Who Erteilt Chinesischem Impfstoff Notfallzulassung Wissen Tagesspiegel

Sinopharm Impfstoff Fur Serbien Chinas Coup Vor Der Haustur Der Eu Politik Tagesspiegel
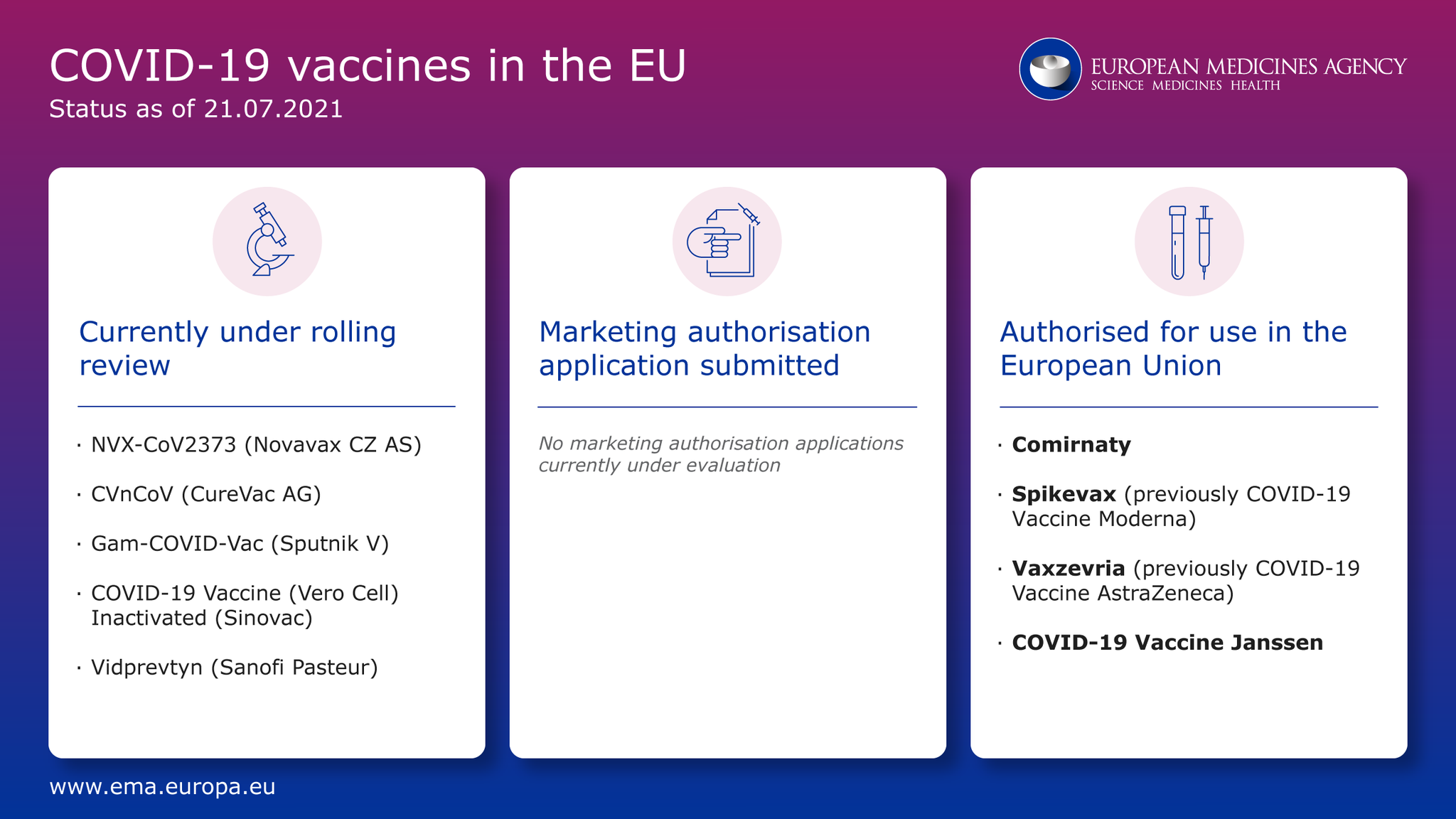
Eu Medicines Agency On Twitter Covid19vaccines Where Are We Now Ema Starts A New Rollingreview For Vidprevtyn Sanofi Pasteur No Marketing Authorisation Application Currently Under Review Https T Co T5aeifknsk

Totimpfstoff Gegen Corona Forderung Nach Neuem Vakzin In Deutschland Gesundheit

Who Erteilt Notfallzulassung Fur Corona Impfstoff Von Sinopharm Der Spiegel

Who Genehmigt Weiteren Impfstoff Aus China Aktuell Welt Dw 01 06 2021
Sinopharm Who Erteilt Notfallzulassung Fur Chinesischen Impfstoff Zeit Online